
Chemistry, 14.02.2022 09:00 whereswoodruff
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(I) nitrate and barium chloride are combined.
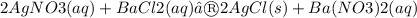
Write the balanced net ionic equation for the reaction.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Compare these two waves : a. the blue wave has a higher pitch, but the orange wave is louder. b.the blue and orange waves have the same volume, but the blue wave has a higher pitch. c.the blue and orange waves have the same pitch, but the blue wave is louder. d.the orange wave has a higher pitch, but the blue wave is louder.
Answers: 1

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions

Mathematics, 17.01.2022 14:00





Mathematics, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00




Mathematics, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00

English, 17.01.2022 14:00

English, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00






