
Chemistry, 14.02.2022 09:50 alexvane78
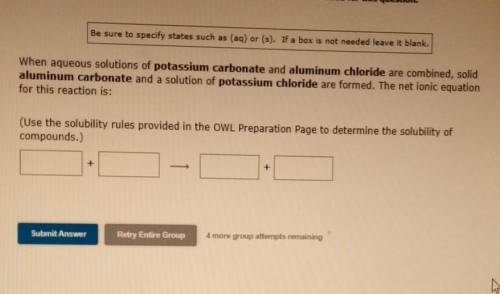
When aqueous solutions of potassium carbonate and aluminum chloride are combined, solid aluminum carbonate and a solution of potassium chloride are formed. The net ionic equation for this reaction is: (Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
When aqueous solutions of potassium carbonate and aluminum chloride are combined, solid aluminum car...
Questions




Mathematics, 04.12.2020 17:20

Biology, 04.12.2020 17:20

Mathematics, 04.12.2020 17:20

Mathematics, 04.12.2020 17:20


Health, 04.12.2020 17:20








Spanish, 04.12.2020 17:20

Computers and Technology, 04.12.2020 17:20


Physics, 04.12.2020 17:20



