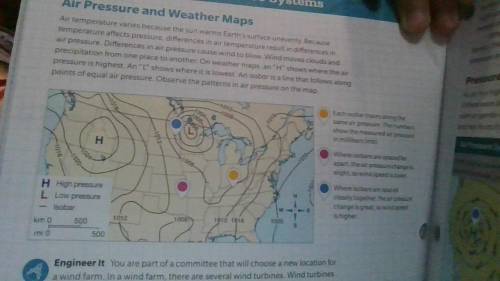
Someone plss help me with this page below...It's due on Monday!
...

Chemistry, 14.02.2022 14:00 tressasill
Someone plss help me with this page below...It's due on Monday!


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

You know the right answer?
Questions



Biology, 23.11.2020 04:20

Mathematics, 23.11.2020 04:20


World Languages, 23.11.2020 04:20





Mathematics, 23.11.2020 04:20

Mathematics, 23.11.2020 04:20


Mathematics, 23.11.2020 04:20

Chemistry, 23.11.2020 04:20




Mathematics, 23.11.2020 04:20

Mathematics, 23.11.2020 04:20



