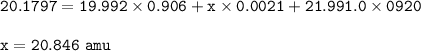Chemistry, 15.02.2022 03:40 jellyangie1
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 amu and an abundance of 90.60% . the second isotope (neon-21) has an abundance of 0.21%. the third isotope has a mass 21.991 amu and an abundance of 9.20%. what is the mass of the second isotope?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 am...
Questions

Mathematics, 03.06.2021 04:00

Mathematics, 03.06.2021 04:00





Social Studies, 03.06.2021 04:00

Mathematics, 03.06.2021 04:10




Social Studies, 03.06.2021 04:10



Computers and Technology, 03.06.2021 04:10

Mathematics, 03.06.2021 04:10

Engineering, 03.06.2021 04:10

Business, 03.06.2021 04:10


Mathematics, 03.06.2021 04:10