
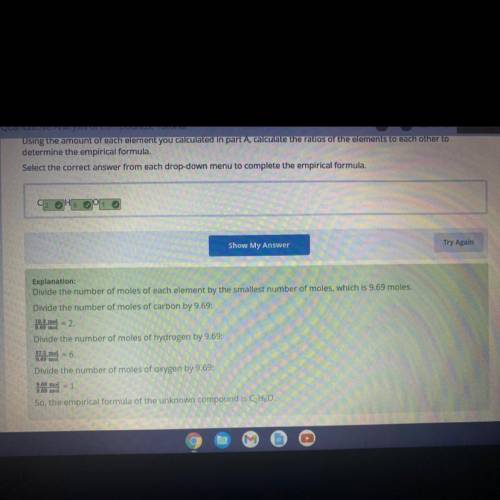
Using the amount of each element you calculated in part A, calculate the ratios of the elements to each other to
determine the empirical formula.
Select the correct answer from each drop-down menu to complete the empirical formula.
CHO
(Answer for those who need help)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1
You know the right answer?
Using the amount of each element you calculated in part A, calculate the ratios of the elements to e...
Questions



Mathematics, 10.03.2020 03:37

Mathematics, 10.03.2020 03:37



Physics, 10.03.2020 03:38


Mathematics, 10.03.2020 03:38



Mathematics, 10.03.2020 03:39

Spanish, 10.03.2020 03:39

Mathematics, 10.03.2020 03:39


Mathematics, 10.03.2020 03:39






