
Chemistry, 18.02.2022 07:00 kordejah348
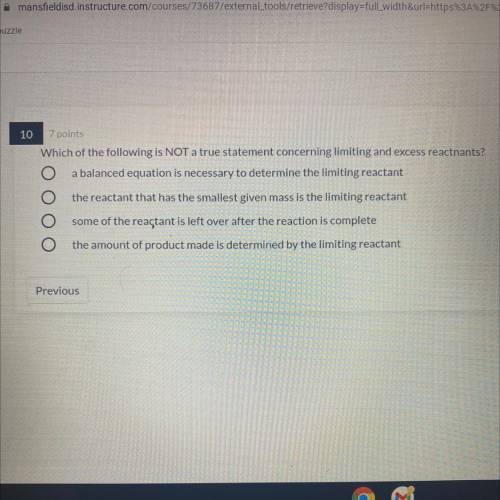
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a balanced equation is necessary to determine the limiting reactant
2.the reactant that has the smallest given mass is the limiting reactant
3. some of the reactant is left over after the reaction is complete
4.the amount of product made is determined by the limiting reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a bal...
Questions

Mathematics, 21.03.2020 21:26


English, 21.03.2020 21:27


Mathematics, 21.03.2020 21:27


Mathematics, 21.03.2020 21:28

Social Studies, 21.03.2020 21:29


Advanced Placement (AP), 21.03.2020 21:29

Spanish, 21.03.2020 21:29




Mathematics, 21.03.2020 21:30




Mathematics, 21.03.2020 21:31




