
Chemistry, 18.02.2022 08:40 arnold2619
How many moles of aluminum are needed to react completely with 4.8 mol of FeO? 2Al(s) + 3Fe(s) - 3e(s) + Al2O3(s)
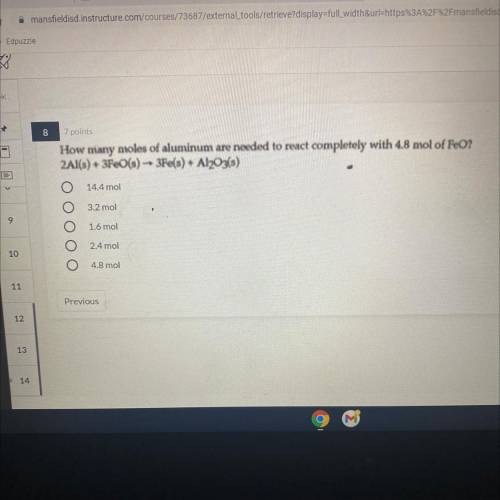

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
How many moles of aluminum are needed to react completely with 4.8 mol of FeO?
2Al(s) + 3Fe(s) - 3...
Questions

Mathematics, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31

History, 20.11.2019 05:31



Mathematics, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31

Physics, 20.11.2019 05:31

English, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31


Chemistry, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31


Chemistry, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31

Mathematics, 20.11.2019 05:31



English, 20.11.2019 05:31



