Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
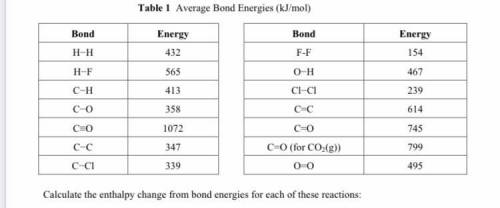
Calculate the enthalpy change from bond energies for each of these reactions:
CO(g) + 2H2(g) → CH3...
Questions

History, 31.08.2021 01:00

Chemistry, 31.08.2021 01:00

Mathematics, 31.08.2021 01:00

History, 31.08.2021 01:00



Mathematics, 31.08.2021 01:00





Mathematics, 31.08.2021 01:00






Arts, 31.08.2021 01:00


Mathematics, 31.08.2021 01:00