
Chemistry, 21.02.2022 07:10 nicolehathaway1012
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
When 447 g of Bi2O3 reacts with excess carbon,
(a) how many moles of Bi form?
(b) how many grams of CO form?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
Questions


Geography, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


Mathematics, 25.11.2021 14:00

Mathematics, 25.11.2021 14:00


Social Studies, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00




Physics, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00



Mathematics, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00

Business, 25.11.2021 14:00

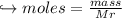
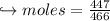
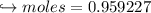
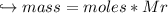
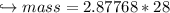
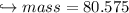 g
g

