1.Which of these statements about the
conservation of mass is not correct?
A. The mass of th...

Chemistry, 24.02.2022 09:10 stalley1521
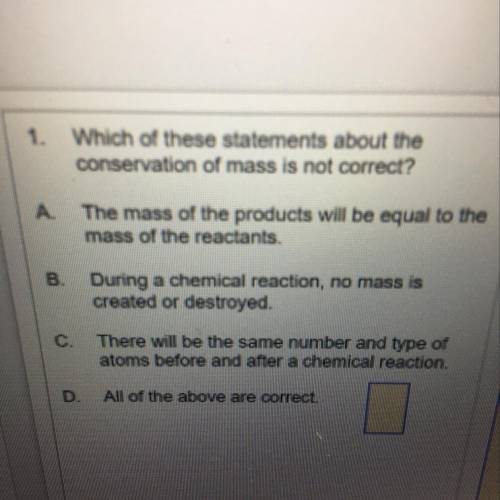
1.Which of these statements about the
conservation of mass is not correct?
A. The mass of the products will be equal to the
mass of the reactants.
B. During a chemical reaction, no mass is
created or destroyed.
C. There will be the same number and type of
atoms before and after a chemical reaction.
D. All of the above are correct.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Questions


Mathematics, 28.04.2021 19:30


Social Studies, 28.04.2021 19:30

History, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30

Mathematics, 28.04.2021 19:30




English, 28.04.2021 19:30


Biology, 28.04.2021 19:30




Mathematics, 28.04.2021 19:30


Computers and Technology, 28.04.2021 19:30

Physics, 28.04.2021 19:30



