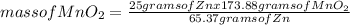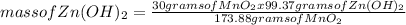Chemistry, 26.02.2022 08:50 sebastianmettsovghhk
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery according to the following reaction: Be sure to check the limiting reactant. Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery...
Questions



Mathematics, 30.01.2020 20:50




Biology, 30.01.2020 20:50

Chemistry, 30.01.2020 20:50

Chemistry, 30.01.2020 20:50


Mathematics, 30.01.2020 20:50

Mathematics, 30.01.2020 20:50



Health, 30.01.2020 20:51

History, 30.01.2020 20:51


Biology, 30.01.2020 20:51


Mathematics, 30.01.2020 20:51