
Chemistry, 06.03.2022 21:50 tressasill
Use the phase diagram for H2O to answer the following questions.
a. What phase is water in at 100°C and 2 atm pressure? (1 point)
b. What happens to water at 100°C as pressure is increased from 0.8 atm to 1.2 atm? (1 point)
c. What happens to water at 1 atm pressure as the temperature is decreased from 10°C to –10°C? (1 point)
d. What line represents boiling points on a phase diagram? (You may describe it or label it on the phase diagram.) (1 point)
e. How do intermolecular forces and kinetic energy interact to determine at what point a liquid will boil? (2 points)
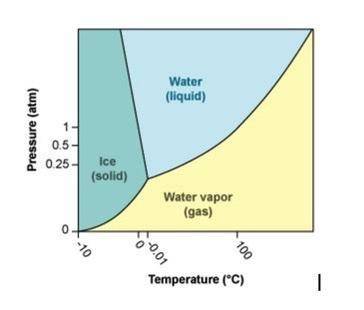

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Use the phase diagram for H2O to answer the following questions.
a. What phase is water in at 100°...
Questions


History, 03.02.2020 15:40


History, 03.02.2020 15:40

History, 03.02.2020 15:40




Social Studies, 03.02.2020 15:40


Spanish, 03.02.2020 15:40






Geography, 03.02.2020 15:43

English, 03.02.2020 15:43

World Languages, 03.02.2020 15:43




