Chemistry, 10.03.2022 06:20 mbatton879
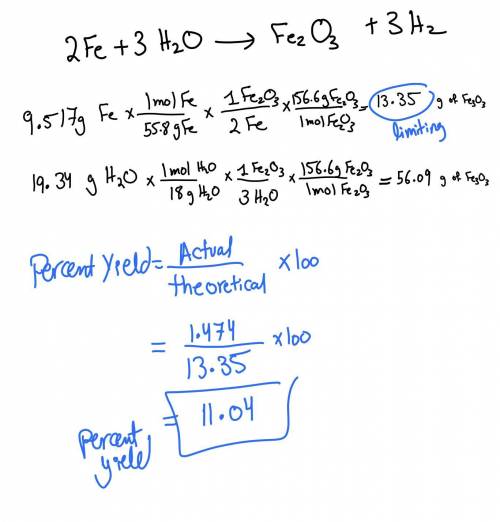
What is the limiting reagent when 9.517 g of Fe is allowed to react with 19.34 g of water according to
the reaction given below? Base your calculations on the yield of Fe2O3. How much of the excess reagent was used and left over. The actual yield of iron(III) oxide was 1.474 g. What is the percent yield?
2 Fe + 3 H20 -› Fe203 + 3 H2

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
What is the limiting reagent when 9.517 g of Fe is allowed to react with 19.34 g of water according...
Questions


Mathematics, 04.05.2021 05:10

Mathematics, 04.05.2021 05:10



Chemistry, 04.05.2021 05:10










English, 04.05.2021 05:10

Mathematics, 04.05.2021 05:10


Mathematics, 04.05.2021 05:10