
Chemistry, 16.03.2022 20:00 kierafisher05
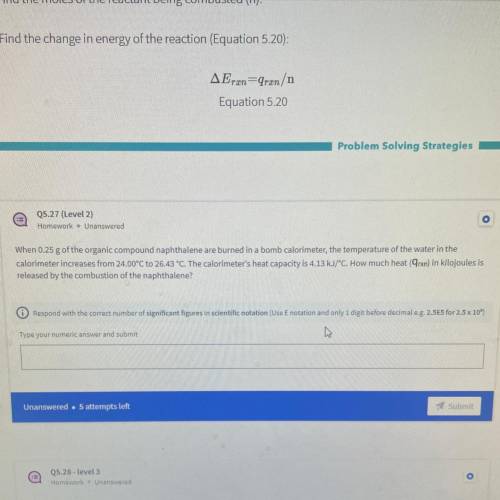
When 0.25 g of the organic compound naphthalene are burned in a bomb calorimeter, the temperature of the water in the
calorimeter increases from 24.00°C to 26.43 °C. The calorimeter's heat capacity is 4.13 kJ/°C. How much heat (9rxn) in kilojoules is
released by the combustion of the naphthalene?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
When 0.25 g of the organic compound naphthalene are burned in a bomb calorimeter, the temperature of...
Questions

Mathematics, 24.04.2020 22:33

English, 24.04.2020 22:33





Physics, 24.04.2020 22:34

Mathematics, 24.04.2020 22:34


History, 24.04.2020 22:34


Mathematics, 24.04.2020 22:34



Spanish, 24.04.2020 22:34


Mathematics, 24.04.2020 22:34

English, 24.04.2020 22:34

Physics, 24.04.2020 22:34



