
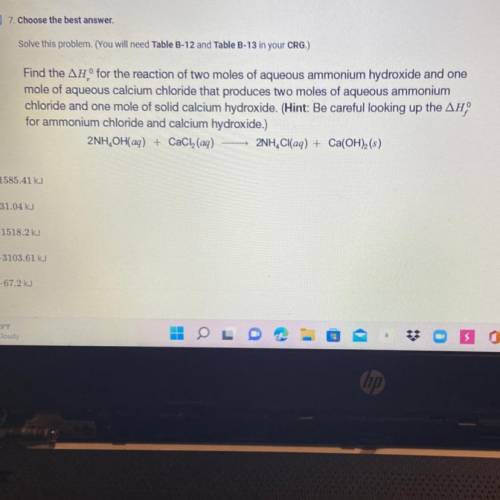
Find the AH° for the reaction of two moles of aqueous ammonium hydroxide and one
mole of aqueous calcium chloride that produces two moles of aqueous ammonium
chloride and one mole of solid calcium hydroxide. (Hint: Be careful looking up the AH
for ammonium chloride and calcium hydroxide.)
2NH, OH(aq) + CaCl(aq) 2NH, Cl(aq) + Ca(OH)(s)
0.-1585.41 kJ
O-31.04 kJ
O-1518.2 kJ
-3103.61 kJ
-67.2 kJ
I NEED HELP FAST !!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1
You know the right answer?
Find the AH° for the reaction of two moles of aqueous ammonium hydroxide and one
mole of aqueous c...
Questions

Mathematics, 30.01.2020 07:48

English, 30.01.2020 07:48


History, 30.01.2020 07:48



Mathematics, 30.01.2020 07:48




Chemistry, 30.01.2020 07:48



Social Studies, 30.01.2020 07:48

Mathematics, 30.01.2020 07:48





Physics, 30.01.2020 07:49



