Carbon disulfide is produced by the reaction listed below:
--->
If you started the...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1


Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1
You know the right answer?
Questions


Spanish, 30.11.2020 20:10

Mathematics, 30.11.2020 20:10

Advanced Placement (AP), 30.11.2020 20:10

Mathematics, 30.11.2020 20:10


Arts, 30.11.2020 20:10

Mathematics, 30.11.2020 20:10


Mathematics, 30.11.2020 20:10




Health, 30.11.2020 20:10

Mathematics, 30.11.2020 20:10

Social Studies, 30.11.2020 20:10

Computers and Technology, 30.11.2020 20:10

Mathematics, 30.11.2020 20:10


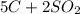 --->
--->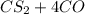
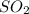 and excess carbon, what amount, in moles, of
and excess carbon, what amount, in moles, of 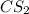 will be produced?
will be produced?

