
Chemistry, 13.04.2022 01:50 tordiacasey
Calculate the enthalpy of reaction for the reaction. C3H8(1) + 502(g) --> 4H2O(g) + 3C02(g) *
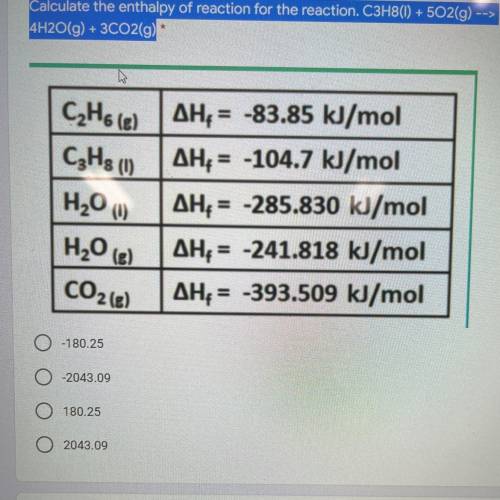

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
Calculate the enthalpy of reaction for the reaction. C3H8(1) + 502(g) --> 4H2O(g) + 3C02(g) *
Questions

Mathematics, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01






Physics, 16.10.2020 20:01




History, 16.10.2020 20:01


Chemistry, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01

History, 16.10.2020 20:01

History, 16.10.2020 20:01



