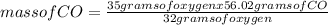When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many grams of co can be formed from 35.0 grams of oxygen? 2c + o2 → 2co using 32.00 g/mole as the molecular mass of oxygen and 28.01 g/mole as the molecular mass of carbon monoxide, solve the above problem.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1


Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many gram...
Questions



Social Studies, 06.10.2019 19:30

Biology, 06.10.2019 19:30

English, 06.10.2019 19:30

Social Studies, 06.10.2019 19:30

Mathematics, 06.10.2019 19:30

Mathematics, 06.10.2019 19:30

Health, 06.10.2019 19:30


Mathematics, 06.10.2019 19:30

Health, 06.10.2019 19:30


History, 06.10.2019 19:30

Biology, 06.10.2019 19:30





English, 06.10.2019 19:30

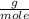 O₂: 32
O₂: 32