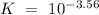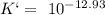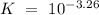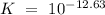Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Which of the compounds above are strong enough acids to react almost completely with a hydroxide ion...
Questions

Mathematics, 16.02.2021 07:10

Spanish, 16.02.2021 07:10




Mathematics, 16.02.2021 07:10


English, 16.02.2021 07:10


Mathematics, 16.02.2021 07:10

Mathematics, 16.02.2021 07:10

Social Studies, 16.02.2021 07:10


Mathematics, 16.02.2021 07:10

Mathematics, 16.02.2021 07:20


Mathematics, 16.02.2021 07:20

English, 16.02.2021 07:20


Spanish, 16.02.2021 07:20

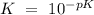
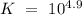
![K`=~10^{1.12]](/tpl/images/0038/9864/3bef3.png)