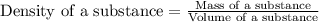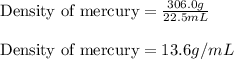Chemistry, 02.07.2019 13:10 oddoneshenchman
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fill the cylinder weighs 306.0 g. from this information, calculate the density of mercury.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fi...
Questions


Mathematics, 05.09.2020 19:01


Chemistry, 05.09.2020 19:01


Mathematics, 05.09.2020 19:01

Social Studies, 05.09.2020 19:01

Social Studies, 05.09.2020 19:01


Computers and Technology, 05.09.2020 19:01






Mathematics, 05.09.2020 19:01

English, 05.09.2020 19:01


Chemistry, 05.09.2020 19:01