

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions

Chemistry, 02.10.2019 15:50





Chemistry, 02.10.2019 15:50

Mathematics, 02.10.2019 15:50

Mathematics, 02.10.2019 15:50

Biology, 02.10.2019 15:50


Mathematics, 02.10.2019 15:50



Mathematics, 02.10.2019 15:50

English, 02.10.2019 15:50

Mathematics, 02.10.2019 15:50

Social Studies, 02.10.2019 15:50

Mathematics, 02.10.2019 15:50


Mathematics, 02.10.2019 15:50

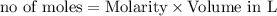
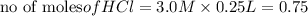
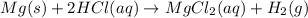
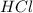 is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
is a limiting reagent as it limits the formation of products and Mg is an excess reagent.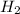
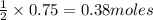 of
of 

