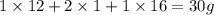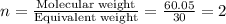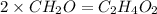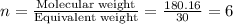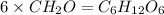The empirical formula for two compounds that have very different properties (one tastes very sour and the other very sweet) is ch2o. if the molar mass of compound a is 60.05 g/mol and compound b is 180.16 g/mol, what are the molecular formulas for these compounds, respectively?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
The empirical formula for two compounds that have very different properties (one tastes very sour an...
Questions

Mathematics, 21.09.2021 08:20

Mathematics, 21.09.2021 08:20



Engineering, 21.09.2021 08:20








Arts, 21.09.2021 08:20

Mathematics, 21.09.2021 08:30

Mathematics, 21.09.2021 08:30



Mathematics, 21.09.2021 08:30

Biology, 21.09.2021 08:30

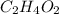
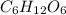
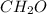 , empirical weight is =
, empirical weight is =