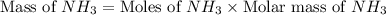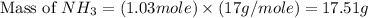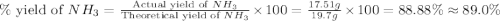Chemistry, 12.10.2019 14:30 jademckinziemea
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent yield of ammonia if the actual yield is 1.03 moles and the theoretical yield is 19.7 grams?
a. 17.5%
b. 35.0%
c. 52.3%
d. 89.0%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent...
Questions

History, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10



Chemistry, 17.11.2020 21:10



Mathematics, 17.11.2020 21:10


Mathematics, 17.11.2020 21:10


Biology, 17.11.2020 21:10

English, 17.11.2020 21:10



Chemistry, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10


Mathematics, 17.11.2020 21:10

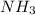 = 1.03 mole
= 1.03 mole