
Chemistry, 02.07.2019 14:50 connorhoran05
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution. be aware of stoichiometry!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Calculate the resulting ph if 365 ml of 2.88 m hno3 is mixed with 335 ml of 1.10 m ca(oh)2 solution....
Questions


History, 20.10.2020 20:01





English, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01




Mathematics, 20.10.2020 20:01





Business, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

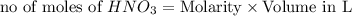
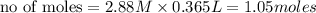
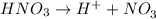
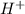 ions = 1.05
ions = 1.05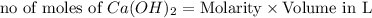
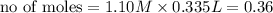
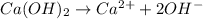
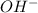 ions =
ions = 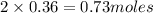
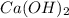 will neutralize 0.73 moles of
will neutralize 0.73 moles of 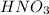 and (1.05-0.73)=0.32 moles of
and (1.05-0.73)=0.32 moles of 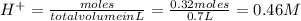
![pH=-\log[H^+]=-\log[0.46]=0.34](/tpl/images/0042/8951/36f67.png)


