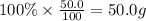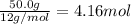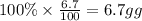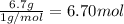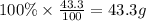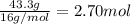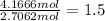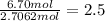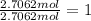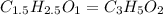Chemistry, 02.07.2019 18:40 pheonixhowls
What is the empirical formula of a compound that contains 50.0% carbon, 6.7% hydrogen, and 43.3% oxygen by mass? ch3o c3h5o2 c2h2o5 c3h205 c1h3o5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

You know the right answer?
What is the empirical formula of a compound that contains 50.0% carbon, 6.7% hydrogen, and 43.3% oxy...
Questions

History, 18.03.2021 02:00





Physics, 18.03.2021 02:00

Business, 18.03.2021 02:00

Physics, 18.03.2021 02:00



Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00






Biology, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

Chemistry, 18.03.2021 02:00

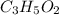 the empirical formula of a compound.
the empirical formula of a compound.