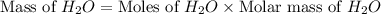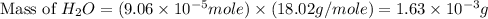Chemistry, 03.07.2019 05:00 rose782751
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 = 32.00 g/mol; molar mass of h2o = 18.02 g/mol) 1.63 × 10-3 g 8.16 × 10-4 g 1.29 × 10-3 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 12:00
In ccl4 is there a certain length that the main atom carbon is a certain distance from the chlorine’s?
Answers: 1
You know the right answer?
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 =...
Questions

Mathematics, 13.06.2021 09:20

Geography, 13.06.2021 09:20

Mathematics, 13.06.2021 09:20


Mathematics, 13.06.2021 09:20


Physics, 13.06.2021 09:20

Biology, 13.06.2021 09:20

Mathematics, 13.06.2021 09:20

Mathematics, 13.06.2021 09:20



Mathematics, 13.06.2021 09:20



Mathematics, 13.06.2021 09:20

Mathematics, 13.06.2021 09:20



Biology, 13.06.2021 09:20

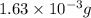
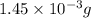
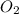 .
.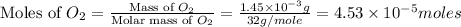
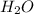 .
.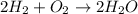
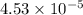 moles of
moles of 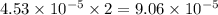 moles of
moles of