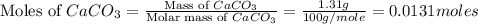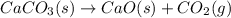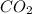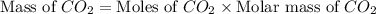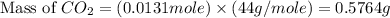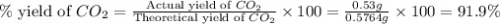Chemistry, 03.07.2019 22:00 laraekeyes
Asample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. what is the percent yield for this reaction? caco3(s) ⟶ cao(s) + co2(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Asample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. what is the...
Questions



Mathematics, 04.12.2020 05:00


Business, 04.12.2020 05:00

Mathematics, 04.12.2020 05:00

Mathematics, 04.12.2020 05:00



Mathematics, 04.12.2020 05:00




Mathematics, 04.12.2020 05:00




Mathematics, 04.12.2020 05:00


Biology, 04.12.2020 05:00

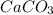 .
.