
Chemistry, 07.07.2019 02:20 allhailkingmilkdud
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2o the acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2. how many moles of co2 are produced when 35.0 mol c2h2 react completely?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2...
Questions

Mathematics, 07.01.2021 01:00

Biology, 07.01.2021 01:00

Social Studies, 07.01.2021 01:00

Advanced Placement (AP), 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00


English, 07.01.2021 01:00




Medicine, 07.01.2021 01:00

Chemistry, 07.01.2021 01:00



Biology, 07.01.2021 01:00

SAT, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

History, 07.01.2021 01:00

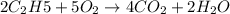
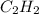 combine with 5 moles of oxygen
combine with 5 moles of oxygen 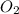 to produce 4 moles of carbon dioxide
to produce 4 moles of carbon dioxide 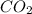 .
.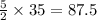 moles of oxygen
moles of oxygen 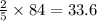 moles of acetylene
moles of acetylene 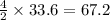 moles of of carbon dioxide
moles of of carbon dioxide 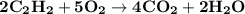
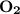 , react with
, react with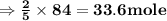 of acetone
of acetone 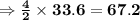 of Carbon dioxide
of Carbon dioxide 

