

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1
You know the right answer?
Give the symbol for the element that has an electron configuration of 1s22s22p63s23p4....
Questions


Biology, 01.12.2021 17:50

Mathematics, 01.12.2021 17:50

Chemistry, 01.12.2021 17:50

Mathematics, 01.12.2021 17:50

Physics, 01.12.2021 17:50



English, 01.12.2021 17:50

Mathematics, 01.12.2021 17:50

Social Studies, 01.12.2021 17:50





English, 01.12.2021 17:50

Spanish, 01.12.2021 17:50

Computers and Technology, 01.12.2021 17:50

Chemistry, 01.12.2021 17:50

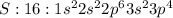
![S:16:[Ne]3s^23p^4](/tpl/images/0063/0807/70407.png)


