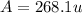Chemistry, 08.07.2019 23:10 KillerSteamcar
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an isotope with a mass of 267.8 u, and 9.7 percent of the sample is an isotope with a mass of 270.9 u. what is the weighted average atomic mass for this element? 269.4 u 268.1 u 269.0 u 270.6 u

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an...
Questions

History, 29.10.2020 17:50



History, 29.10.2020 17:50

Spanish, 29.10.2020 17:50


English, 29.10.2020 17:50

History, 29.10.2020 17:50


Mathematics, 29.10.2020 17:50


History, 29.10.2020 17:50


Computers and Technology, 29.10.2020 17:50


Mathematics, 29.10.2020 17:50

Mathematics, 29.10.2020 17:50


Social Studies, 29.10.2020 17:50

Computers and Technology, 29.10.2020 17:50

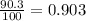
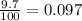
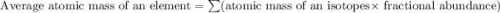
![A=\sum[267.8\times 0.903+270.9\times 0.097]](/tpl/images/0067/2857/bf750.png)