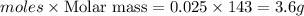Chemistry, 09.07.2019 16:20 heroicblad
Silver nitrate and aluminum chloride react with each other by exchanging anions: 3agno3 (aq) + alcl3 (aq) → al(no3)3 (aq) + 3agcl (s) what mass in grams of agcl is produced when 4.22 g of agno3 react with 7.73 g of alcl3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Silver nitrate and aluminum chloride react with each other by exchanging anions: 3agno3 (aq) + alcl...
Questions








Social Studies, 23.07.2019 16:30

Mathematics, 23.07.2019 16:30


History, 23.07.2019 16:30



Chemistry, 23.07.2019 16:30

Mathematics, 23.07.2019 16:30


History, 23.07.2019 16:30

Chemistry, 23.07.2019 16:30


Mathematics, 23.07.2019 16:30

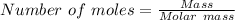
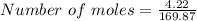
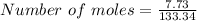
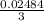 moles of AlCl₃ completely.
moles of AlCl₃ completely. 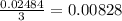
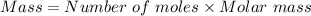
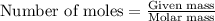
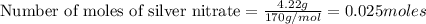
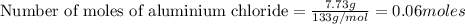
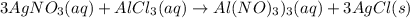
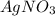 react with 1 mole of
react with 1 mole of 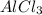
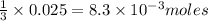 of
of 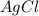
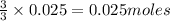 of
of