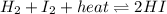Chemistry, 09.07.2019 19:00 tddreviews
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemical equilibrium if the temperature of the system is increased? a)the direction of the chemical equilibrium will shift to the right, favoring the forward reaction. b)the chemical equilibrium will not be affected by an increase in temperature. c)the direction of the chemical equilibrium will shift to the left, favoring the reverse reaction. d) the chemical equilibrium will be lost permanently with a change of temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

You know the right answer?
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemic...
Questions


Mathematics, 12.10.2019 19:10



Mathematics, 12.10.2019 19:10

Mathematics, 12.10.2019 19:10




English, 12.10.2019 19:10

Mathematics, 12.10.2019 19:10

English, 12.10.2019 19:10




Mathematics, 12.10.2019 19:10


Mathematics, 12.10.2019 19:10