
Chemistry, 20.08.2019 03:00 dheydar9377
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final reading is 38.55 ml. using the correct number of significant figures, determine the density of the metal = g/ml first determine volume of metal: report result to the same number of decimal places as the number with the least number of decimal places. use this volume in calculation. second determine density of metal: d=m/v report result to the same number of significant figures as the number with the least number of significant figures. do not use exponential notation in answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3
You know the right answer?
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final rea...
Questions

Mathematics, 23.10.2020 02:01


Physics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

English, 23.10.2020 02:01


English, 23.10.2020 02:01

History, 23.10.2020 02:01



Mathematics, 23.10.2020 02:01



History, 23.10.2020 02:01


Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

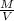 =
= 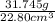 = 1.392 g/cm³
= 1.392 g/cm³

