
Chemistry, 20.08.2019 21:30 rfcordray307
For co2 the van der waals constants are a=3.59 atm • l2/mol2 and b=0.0427 l/mol. calculate the pressure exerted by 2.50 moles of co2 confined in a volume of 5.00 l at 450 k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
For co2 the van der waals constants are a=3.59 atm • l2/mol2 and b=0.0427 l/mol. calculate the press...
Questions


Mathematics, 26.07.2021 23:50

Mathematics, 26.07.2021 23:50

Mathematics, 26.07.2021 23:50



Spanish, 26.07.2021 23:50


Mathematics, 26.07.2021 23:50

Mathematics, 26.07.2021 23:50

Mathematics, 26.07.2021 23:50


English, 26.07.2021 23:50

Spanish, 26.07.2021 23:50

Law, 26.07.2021 23:50


Social Studies, 27.07.2021 01:00

Law, 27.07.2021 01:00


English, 27.07.2021 01:00

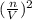 ](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²
](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²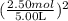 ](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm =
](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm = 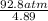 = 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atm
= 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atm

