

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

You know the right answer?
What is the specific heat of metal if its mass is 26.86g and it requires 418.6 j of heat energy to r...
Questions

Biology, 19.07.2019 16:50

Biology, 19.07.2019 16:50

Business, 19.07.2019 16:50

Mathematics, 19.07.2019 16:50

Chemistry, 19.07.2019 16:50

Social Studies, 19.07.2019 16:50

History, 19.07.2019 16:50

Biology, 19.07.2019 16:50

Biology, 19.07.2019 16:50

Social Studies, 19.07.2019 16:50



History, 19.07.2019 16:50

Health, 19.07.2019 16:50





History, 19.07.2019 16:50

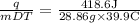 = 0.364 J·°C⁻¹·g⁻¹
= 0.364 J·°C⁻¹·g⁻¹

