At normal temperature and pressure, air has
a density of 0.001184 g/ml. what is the mass
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1


You know the right answer?
Questions

Mathematics, 04.11.2019 00:31

Mathematics, 04.11.2019 00:31


Mathematics, 04.11.2019 00:31




English, 04.11.2019 00:31


Mathematics, 04.11.2019 00:31

Advanced Placement (AP), 04.11.2019 00:31

History, 04.11.2019 00:31




English, 04.11.2019 00:31

Mathematics, 04.11.2019 00:31

Mathematics, 04.11.2019 00:31


Mathematics, 04.11.2019 00:31

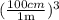 ×
× 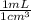 = 3.655 × 10⁷ mL
= 3.655 × 10⁷ mL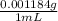 = 43 300 g = 43.3 kg
= 43 300 g = 43.3 kg

