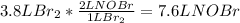Chemistry, 12.07.2019 06:00 Hellokittyjam35
For the following reaction, what volume of nobr can be produced from 3.8 l of br2 (measured at the same temperature and pressure), assuming an excess of no? 2no(g)+br2(g)=2no(br)2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3


Chemistry, 23.06.2019 17:30
If 2.40 moles of gas are held at a temperature of 97.0 °c in a container with a volume of 45.0 l, what is the pressure of the gas?
Answers: 1
You know the right answer?
For the following reaction, what volume of nobr can be produced from 3.8 l of br2 (measured at the s...
Questions

Mathematics, 28.07.2019 09:50


Chemistry, 28.07.2019 09:50

Physics, 28.07.2019 09:50






History, 28.07.2019 09:50



Mathematics, 28.07.2019 09:50


English, 28.07.2019 09:50





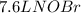
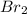 per 2 moles of
per 2 moles of 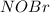 in terms of volume as shown below because of the Avogadro's law (change in mole proportional to the change in volume at constant both pressure and temperature):
in terms of volume as shown below because of the Avogadro's law (change in mole proportional to the change in volume at constant both pressure and temperature):