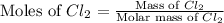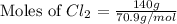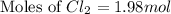Chemistry, 12.07.2019 11:00 honestty21
In the gaseous state, chlorine exists as a diatomic molecule cl2 (molar mass = 70.9 g/mol).calculate the number of moles of chlorine present in 140 g of chlorine gas.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
In the gaseous state, chlorine exists as a diatomic molecule cl2 (molar mass = 70.9 g/mol).calculate...
Questions



Biology, 10.12.2021 07:00



Mathematics, 10.12.2021 07:00

Mathematics, 10.12.2021 07:00

English, 10.12.2021 07:00

History, 10.12.2021 07:00

Biology, 10.12.2021 07:00

French, 10.12.2021 07:00

Mathematics, 10.12.2021 07:00


Biology, 10.12.2021 07:00




Mathematics, 10.12.2021 07:00

Mathematics, 10.12.2021 07:00

Mathematics, 10.12.2021 07:00

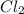 = 140 g
= 140 g