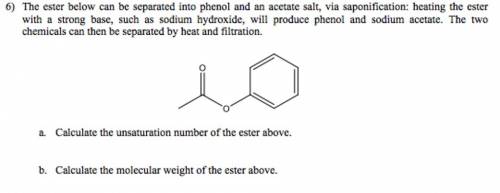
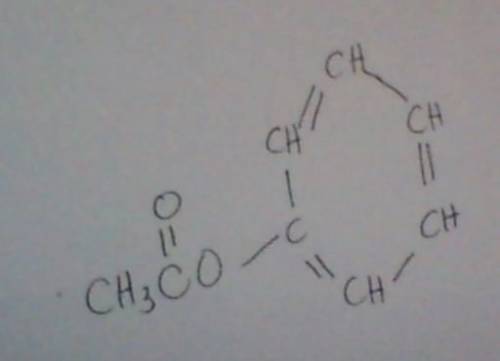
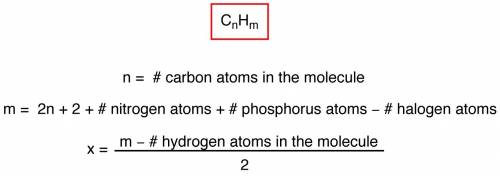
The ester below can be separated into phenol and an acetate salt, via saponification: heating the ester with a strong base, such as sodium hydroxide, will produce phenol and sodium acetate. the two chemicals can then be separated by heat and filtration. a. calculate the unsaturation number of the ester above. b. calculate the molecular weight of the ester above

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2
You know the right answer?
The ester below can be separated into phenol and an acetate salt, via saponification: heating the e...
Questions



History, 28.08.2019 04:00

Mathematics, 28.08.2019 04:00




Computers and Technology, 28.08.2019 04:00


English, 28.08.2019 04:00


History, 28.08.2019 04:00


Computers and Technology, 28.08.2019 04:00


Physics, 28.08.2019 04:00

History, 28.08.2019 04:00


English, 28.08.2019 04:00