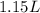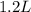Asyringe contains 0.65 moles of he gas that occupy 750.0 ml. what volume (in l) of gas will the syringe hold if 0.35 moles of ne is added? a syringe contains 0.65 moles of he gas that occupy 750.0 ml. what volume (in l) of gas will the syringe hold if 0.35 moles of ne is added? 1.9 l 4.9 l 2.1 l 0.87 l 1.2 l

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
Asyringe contains 0.65 moles of he gas that occupy 750.0 ml. what volume (in l) of gas will the syri...
Questions


Mathematics, 18.11.2020 23:40



History, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

English, 18.11.2020 23:40




Chemistry, 18.11.2020 23:40




Mathematics, 18.11.2020 23:40


Mathematics, 18.11.2020 23:40


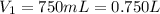
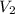
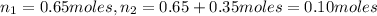
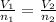
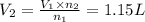
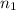 =
= 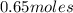 The volume it occupies
The volume it occupies 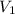 =
= 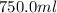 =
= 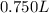 Mole of Neon =
Mole of Neon = 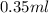
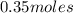 =
= 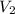 (unknown)
(unknown)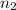 =
= 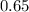 +
+ 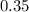 =
= 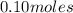 ,
, 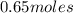
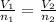
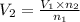
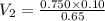 =
=