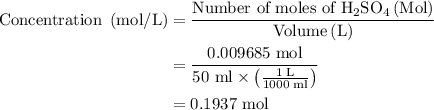Chemistry, 13.07.2019 10:40 kuddlebugsmommy
In an acid-base neutralization reaction 38.74 ml of 0.500 m potassium hydroxide (ki) reacts with 50.00 ml of sulfuric acid solution. what is the concentration of the h2so4 solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
In an acid-base neutralization reaction 38.74 ml of 0.500 m potassium hydroxide (ki) reacts with 50....
Questions



Mathematics, 26.01.2021 03:40

Mathematics, 26.01.2021 03:40

World Languages, 26.01.2021 03:40


Physics, 26.01.2021 03:40



Engineering, 26.01.2021 03:40


Mathematics, 26.01.2021 03:40





Mathematics, 26.01.2021 03:40


English, 26.01.2021 03:40

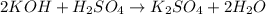
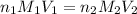
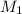 = molarity of acid
= molarity of acid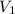 = volume of acid
= volume of acid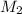 = molarity of base
= molarity of base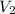 = volume of base
= volume of base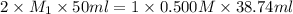
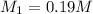
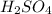 solution is 0.19M.
solution is 0.19M.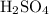 solution is
solution is  .
.
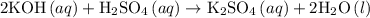
 and
and 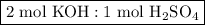
 as follows:
as follows: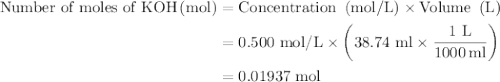
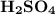 required to neutralize 0.01937 moles of
required to neutralize 0.01937 moles of