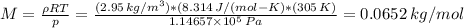Chemistry, 13.07.2019 19:00 KassandraVillegas
What is the molar mass of a pure gaseous compound having a density of 2.95 g/l at 32⁰c and 860 mm hg? (1 atm = 760 mm hg)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
What is the molar mass of a pure gaseous compound having a density of 2.95 g/l at 32⁰c and 860 mm hg...
Questions


SAT, 23.02.2022 02:00

Mathematics, 23.02.2022 02:00



History, 23.02.2022 02:00

Computers and Technology, 23.02.2022 02:10


Social Studies, 23.02.2022 02:10


History, 23.02.2022 02:10


Computers and Technology, 23.02.2022 02:10






Mathematics, 23.02.2022 02:10