

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
A0.8838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolv...
Questions




English, 14.12.2020 23:50






Mathematics, 14.12.2020 23:50



Mathematics, 14.12.2020 23:50

Mathematics, 14.12.2020 23:50

Social Studies, 14.12.2020 23:50

Arts, 14.12.2020 23:50

Mathematics, 14.12.2020 23:50



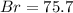 %
%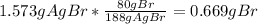
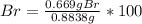 %
%

