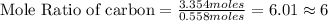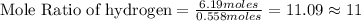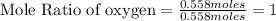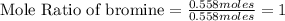Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Tear gas has the composition 40.25% carbon, 6.19% hydrogen, 8.94% oxygen, 44.62% bromine. what is th...
Questions




Mathematics, 28.08.2020 01:01

Chemistry, 28.08.2020 01:01

Mathematics, 28.08.2020 01:01


Chemistry, 28.08.2020 01:01


Mathematics, 28.08.2020 01:01




Computers and Technology, 28.08.2020 01:01






Mathematics, 28.08.2020 01:01

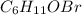
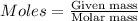
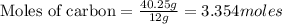 (Molar Mass of C = 12g)
(Molar Mass of C = 12g)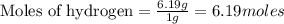 (Molar Mass of H = 1g)
(Molar Mass of H = 1g)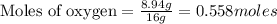 (Molar Mass of O = 16g)
(Molar Mass of O = 16g)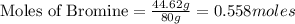 (Molar Mass of Br = 80g)
(Molar Mass of Br = 80g)