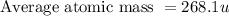Chemistry, 16.07.2019 00:10 aprilpendergrass
Anewly discovered element, y, has two naturally occurring isotopes. 87.8 percent of the sample is an isotope with a mass of 267.8 u, and 12.2 percent of the sample is an isotope with a mass of 269.9 u. what is the weighted average atomic mass for this element? 267.9 u 268.1 u 268.9 u 269.1 u

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 87.8 percent of the sample is an...
Questions





History, 04.01.2022 19:50


Mathematics, 04.01.2022 19:50


English, 04.01.2022 19:50

Mathematics, 04.01.2022 19:50


SAT, 04.01.2022 19:50

Mathematics, 04.01.2022 19:50


History, 04.01.2022 20:00

Spanish, 04.01.2022 20:00


Biology, 04.01.2022 20:00



![\text{Average atomic mass }=[(267.8\times 0.878)+(269.9\times 0.122)]](/tpl/images/0094/3451/ba97e.png)