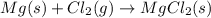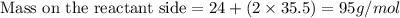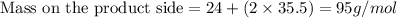Chemistry, 16.07.2019 11:50 savannabroyhill24
Which chemical equation shows that the total mass during a chemical reaction stays the same? a) mg + cl2 → mgcl2 b) naoh + mgcl2 → nacl + mgoh c) 2na + 2h2o → naoh + h2 d) h2o + o2 → h2o

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Which chemical equation shows that the total mass during a chemical reaction stays the same? a) mg...
Questions

History, 03.06.2021 16:50

Mathematics, 03.06.2021 16:50




Mathematics, 03.06.2021 16:50


Mathematics, 03.06.2021 16:50

Chemistry, 03.06.2021 16:50


Chemistry, 03.06.2021 16:50


Mathematics, 03.06.2021 16:50


Mathematics, 03.06.2021 16:50


History, 03.06.2021 16:50

English, 03.06.2021 16:50