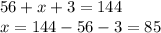Chemistry, 17.07.2019 08:00 rosebelll4555
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b) 85 c) 141 d) 143 the atomic number is consider a unique atom and element identifier. describe the associated subatomic particle, and how it can be used to identify the atom. a) the number of electrons changes in a unique way as the charge on the atom changes. b) the proton number remains constant through changing the atomic charge, energy, and isotopes. c) as the atom absorbs photons the way the photons interact in the nucleus gives the atomic number. d) all isotopes of the an element have the same number of neutrons allowing it to describe the unique atom.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1
You know the right answer?
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b)...
Questions

Advanced Placement (AP), 19.10.2021 19:00


English, 19.10.2021 19:00

Mathematics, 19.10.2021 19:00

Mathematics, 19.10.2021 19:00

Social Studies, 19.10.2021 19:00



Mathematics, 19.10.2021 19:00





Advanced Placement (AP), 19.10.2021 19:00




Mathematics, 19.10.2021 19:00