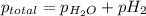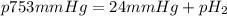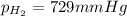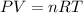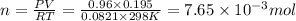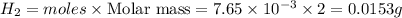Chemistry, 18.07.2019 10:30 brainfreeze911ovksah
Small quantities of h2 gas can be collected by adding hcl to zn. a sample of 195 ml of h2 gas was collected over water at 25 c and 753 mm of hg. what mass of h2 was collected? (vp of water at 25 c is 24 mm of hg)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Small quantities of h2 gas can be collected by adding hcl to zn. a sample of 195 ml of h2 gas was co...
Questions

Computers and Technology, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

History, 23.10.2020 16:40


Chemistry, 23.10.2020 16:40



English, 23.10.2020 16:40


English, 23.10.2020 16:40



Chemistry, 23.10.2020 16:40



Mathematics, 23.10.2020 16:40


Mathematics, 23.10.2020 16:40


Health, 23.10.2020 16:40