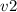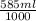Chemistry, 18.07.2019 11:00 izzy123abc
Asyringe contains 0.65 moles of he gas that occupy 900.0 ml. what volume (in l) of gas will the syringe hold if 0.35 moles of ne is added?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Asyringe contains 0.65 moles of he gas that occupy 900.0 ml. what volume (in l) of gas will the syri...
Questions

Mathematics, 12.10.2020 05:01

Social Studies, 12.10.2020 05:01

Physics, 12.10.2020 05:01



World Languages, 12.10.2020 05:01

Biology, 12.10.2020 05:01

Computers and Technology, 12.10.2020 05:01

Mathematics, 12.10.2020 05:01

Mathematics, 12.10.2020 05:01




Arts, 12.10.2020 05:01





Mathematics, 12.10.2020 05:01

History, 12.10.2020 05:01

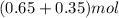
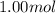
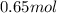 x
x 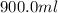 =
= 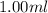 x
x 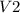
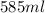 =
= 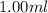 x
x