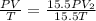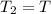Chemistry, 18.07.2019 17:40 ernestscott406
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p, and its temperature is t. if the balloon is heated to a temperature of 15.5t while it is placed under a high pressure of 15.5p, how does the volume of the balloon change?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

You know the right answer?
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p...
Questions



History, 27.10.2019 11:43

Mathematics, 27.10.2019 11:43



History, 27.10.2019 11:43


History, 27.10.2019 11:43

Biology, 27.10.2019 11:43

Mathematics, 27.10.2019 11:43


English, 27.10.2019 11:43


Biology, 27.10.2019 11:43




Mathematics, 27.10.2019 11:43

Mathematics, 27.10.2019 11:43

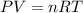
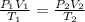
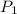 = initial pressure
= initial pressure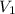 = initial volume
= initial volume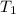 = initial temperature
= initial temperature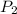 = final pressure
= final pressure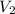 = final volume
= final volume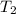 = final temperature
= final temperature